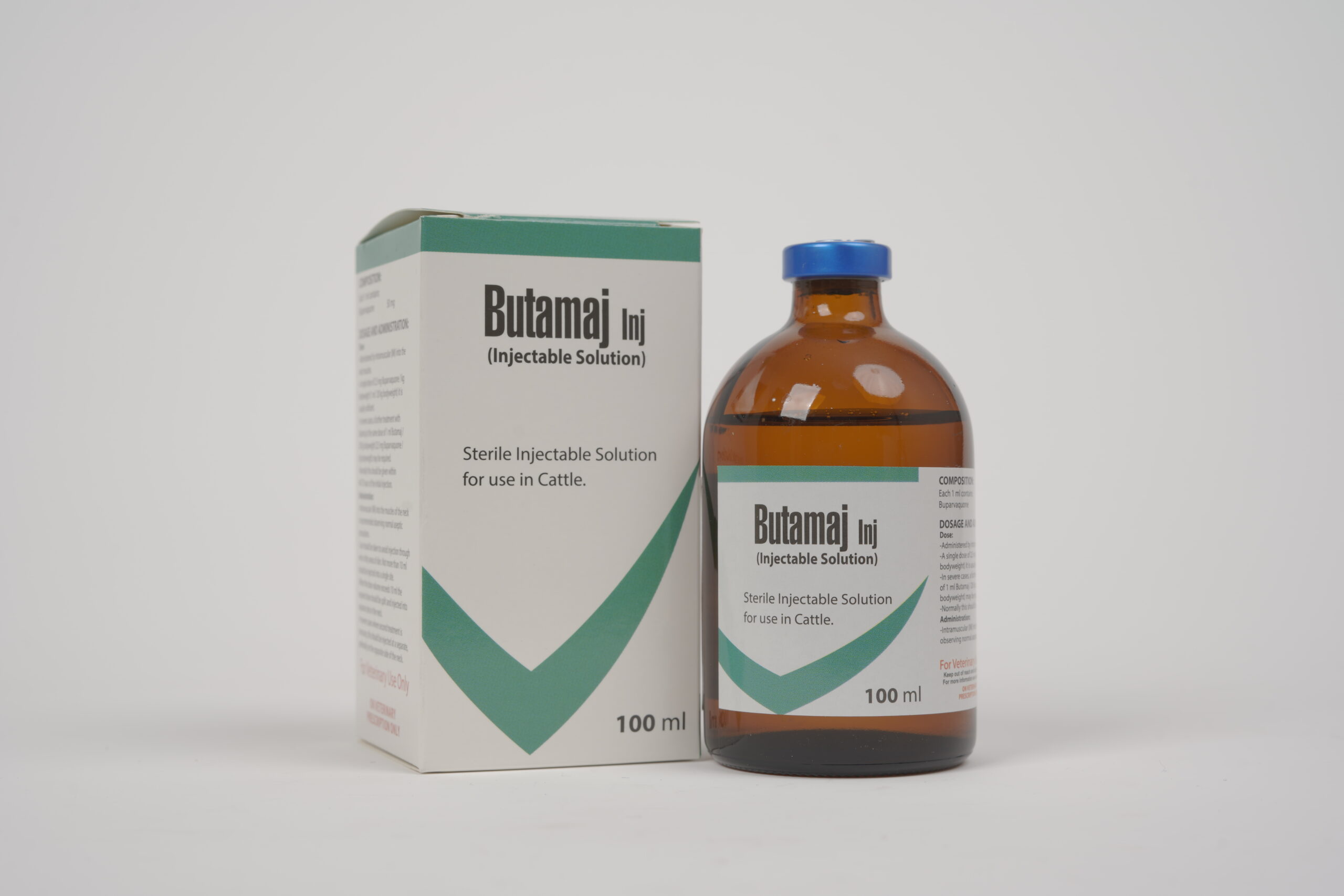
Butamaj Inj.
0
People watching this product now!
Description
Description
Composition:
Each 1 ml contains:
Buparvaquone : 50 mg
Properties:
- Buparvaquone is a second-generation hydroxynaphthoquinone related to parvaquone, its used for the therapy and prophylaxis of all forms of theileriosis.
- Long plasma half-life (at least 7 days).
- Low toxicity: oral LD50 in rats > 8000 mg/kg
Indications:
- Butamaj Inj. is indicated for the treatment of theileriosis (East Coast Fever, Corridor disease, Tropical theileriosis, etc) in cattle caused by Theileria parva, T. annulata, T. mutans, and T. orientalis.
- It is active against both the Schizonts and piroplasms stages of theileria and may be used during the incubation period of the disease or when clinical signs are apparent.
Dosage and administration:
Administered by intramuscular injection into the neck muscles.
Butamaj Inj. is administered as a single dose at the rate of 1ml per 20kg bodyweight (equivalent to 2.5mg buparvaquone/ kg bodyweight).
- In severe and advanced cases, a second treatment may be required at 48-72 hours after the initial injection at the same dose rate.
- Avoid wet or dirty areas of the skin.
- Not more than 10 ml should be injected into a single site.
Warnings and Precautions:
- Butamaj Inj. should never be given by intravenous or subcutaneous injection.
- Localised, painless, oedematous swelling may occasionally be at the injection site.
- Theileriosis has severe depressant effects on the immune system. Therefore, it is recommended that any vaccination be delayed until the animal has recovered.
Withdrawal period:
- Animals should not be slaughtered for human consumption during treatment and within 42 days from the last treatment.
- Milk from treated cattle should not be used for human consumption during treatment and within 48 hours from the last treatment.
Storage:
Store at a temperature below 30oC.
Reviews (0)
Be the first to review “Butamaj Inj.” Cancel reply








Reviews
There are no reviews yet.